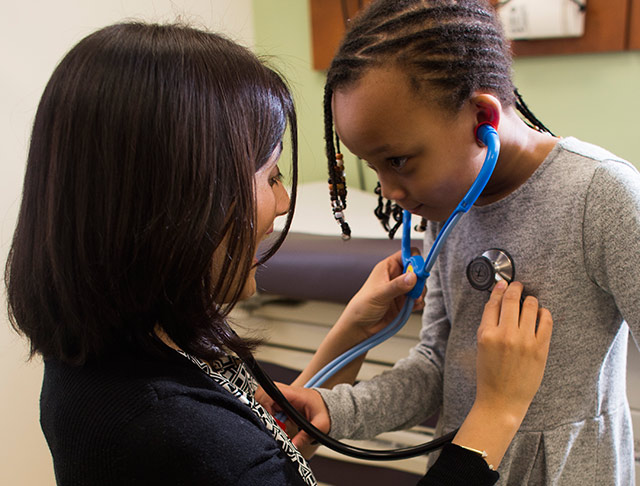Mount Sinai Named to Modern Healthcare’s 2024 Innovators List
The list recognizes leaders and organizations pioneering innovative transformations in health care.

The Mount Sinai Hospital Ranked No. 1 in New York by Newsweek/Statista
In the latest rankings, we are among the best everywhere—No. 1 in New York City, No. 8 nationally, and No. 20 in the world.

Advancing Heart Care For Families
Our award-winning cardiology teams are working towards important advancements in understanding hearts better.
.jpg)
Expert Cancer Care You Can Rely On
Our team works relentlessly to offer the most accurate diagnosis, the latest clinical trials, and the best treatment options.
2023-2024 Mount Sinai Science & Medicine Magazine
How Mount Sinai Integrates Cutting-Edge Technology With Patient-Centered Expertise

Mount Sinai Health System Names Brendan Carr, MD, MA, MS, as Next Chief Executive Officer
Nationally recognized leader in academic medicine, delivering high-quality health care as a physician, health policy researcher, and educator.

Digital Patient Tools and Resources
See how our expert care is always there
Getting care is easy, whether you choose in-person or virtual care.
Book an Appointment now with a Mount Sinai doctor.
Sign in with your MyChart account and take care of your healthcare needs.
Call us at 1-800-MD-SINAI

Despite AI Advancements, Human Oversight Remains Essential
Apr 22, 2024 View All Press Releases
Mount Sinai Health System Named to Modern Healthcare’s 2024 Innovators list
Apr 09, 2024 View All Press Releases
Inter-Atrial Shunts May Benefit Some Heart Failure Patients While Harming Others
Apr 06, 2024 View All Press Releases