Mount Sinai Researchers Review Progress of Treating Glutamate Signalling in Depression
Major depressive disorder (MDD) impacts 15 million Americans and is the leading cause of disability, yet current treatments possess limited efficacy. Ketamine, which has been repurposed as a rapidly acting antidepressant, has emerged as an experimental and potentially promising compound to treat severe depression through a novel drug action mechanism that blocks glutamate receptors.
Researchers from the Icahn School of Medicine at Mount Sinai and other institutions reviewed progress made in using ketamine and other therapies to treat depression. While they found that glutamate modulating agents including ketamine may represent the first major advance in treating MDD in decades, fundamental questions remain regarding safety, tolerability, and efficacy. The review will be published online, March 17, in Nature Reviews / Drug Discovery.
Neurotransmitters, including glutamate, are substances that conduct signals from one nerve cell to another. Glutamate is the most abundant excitatory neurotransmitter that promotes the flow of signals between nerve cells. Mental disorders such as depression and schizophrenia stem in part from an inability of the central nervous system to effectively use glutamate. Experimental drugs that block glutamate receptors in the central nervous system or lower glutamate brain levels may represent the next generation of antidepressant medications, and offer potential advantages over current drug treatments.
“The ongoing clinical trial research focusing on the glutamate system may lead to a completely new class of antidepressants that may significantly change the way patients with depression, and in particular, treatment-resistant depression are treated,” said the study’s first author, James Murrough, MD, Director, Mood and Anxiety Disorders Program and Assistant Professor of Psychiatry and Neuroscience at the Icahn School of Medicine at Mount Sinai. “Targeting glutamate receptors could transform care for patients for this devastating disease.”
Researchers at Mount Sinai’s Mood and Anxiety Disorders Program (MAP) are conducting studies and clinical trials on agents, including ketamine, which modulate the glutamate system. No glutamate modulator has been approved for the treatment of depression worldwide.
Ketamine, a schedule III controlled substance drug with a potential for abuse, has been studied in patients with depression. The drug has been given in low doses and in a controlled environment.
The review identifies key outstanding issues related to the development of ketamine and other glutamate modulators for use in depression. For example, most studies have selected patients who have failed to respond to one or more trials of conventional antidepressants and have not accounted for other factors, including trauma history or genetic predisposition for depression.
“Although substantial progress has been made over the past decade in identifying ketamine as a prototype rapid-acting antidepressant, there is a large gap in the literature, which represents a crucial unmet research need to examine its safety and efficacy beyond a single treatment administration,” said Dr. Murrough. “An important unknown is the relationship between glutamate modulation and conventional therapeutic approaches for depression, which may shed light on the strengths and limitations of this approach.”
The Icahn School of Medicine at Mount Sinai is a co-owner of a patent covering the use of ketamine for the treatment of treatment-resistant depression, has entered into a licensing agreement with a pharmaceutical company for rights to the patent, and will receive payments related to the use of ketamine if it is approved for the treatment of treatment-resistant depression. Dr. Murrough is not named as an inventor on the patent and will not receive any payments.
About the Mount Sinai Health System
Mount Sinai Health System is one of the largest academic medical systems in the New York metro area, with more than 43,000 employees working across eight hospitals, over 400 outpatient practices, nearly 300 labs, a school of nursing, and a leading school of medicine and graduate education. Mount Sinai advances health for all people, everywhere, by taking on the most complex health care challenges of our time — discovering and applying new scientific learning and knowledge; developing safer, more effective treatments; educating the next generation of medical leaders and innovators; and supporting local communities by delivering high-quality care to all who need it.
Through the integration of its hospitals, labs, and schools, Mount Sinai offers comprehensive health care solutions from birth through geriatrics, leveraging innovative approaches such as artificial intelligence and informatics while keeping patients’ medical and emotional needs at the center of all treatment. The Health System includes approximately 7,300 primary and specialty care physicians; 13 joint-venture outpatient surgery centers throughout the five boroughs of New York City, Westchester, Long Island, and Florida; and more than 30 affiliated community health centers. We are consistently ranked by U.S. News & World Report's Best Hospitals, receiving high "Honor Roll" status, and are highly ranked: No. 1 in Geriatrics and top 20 in Cardiology/Heart Surgery, Diabetes/Endocrinology, Gastroenterology/GI Surgery, Neurology/Neurosurgery, Orthopedics, Pulmonology/Lung Surgery, Rehabilitation, and Urology. New York Eye and Ear Infirmary of Mount Sinai is ranked No. 12 in Ophthalmology. U.S. News & World Report’s “Best Children’s Hospitals” ranks Mount Sinai Kravis Children's Hospital among the country’s best in several pediatric specialties.
For more information, visit https://www.mountsinai.org or find Mount Sinai on Facebook, Twitter and YouTube.
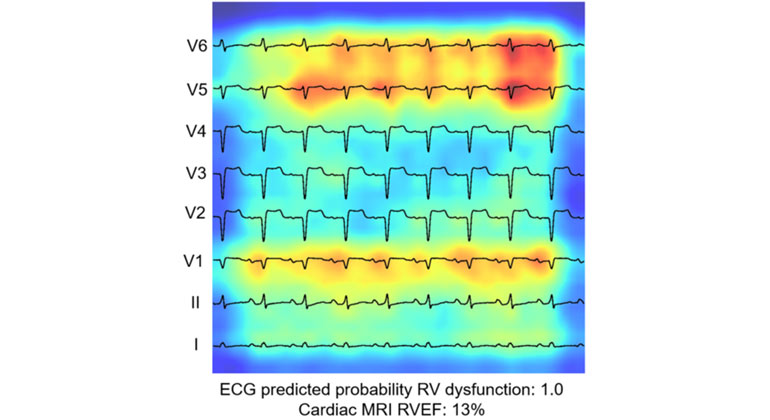
AI-Driven Study Redefines Right Heart Health Assessment With Novel Predictive Model
Jan 04, 2024 View All Press Releases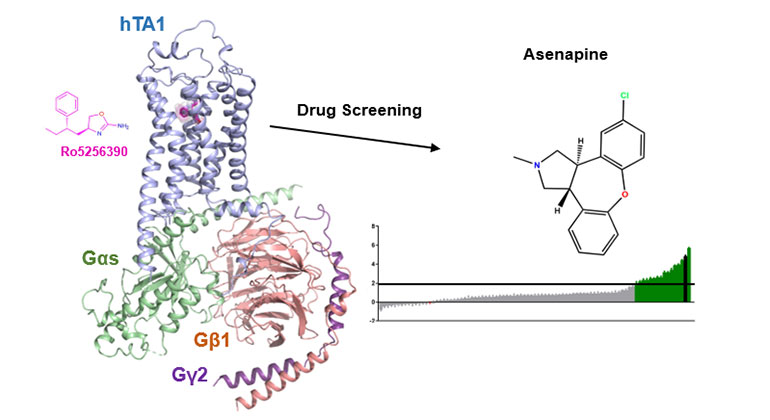
Demystifying a Key Receptor in Substance Use and Neuropsychiatric Disorders
Jan 02, 2024 View All Press Releases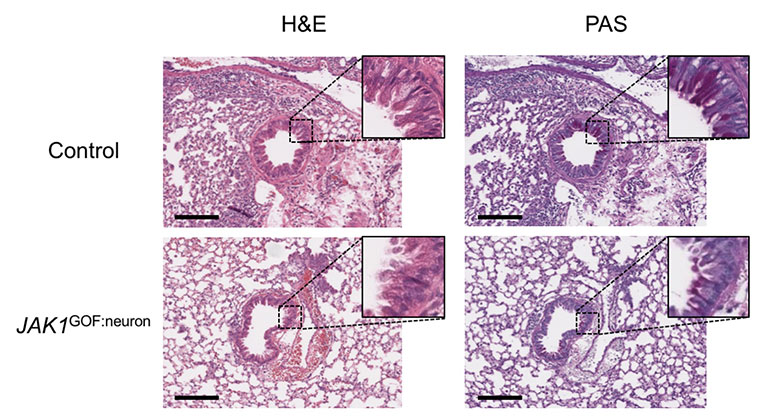
New Insights Revealed On Tissue-Dependent Roles of JAK Signaling in Inflammation
Dec 21, 2023 View All Press Releases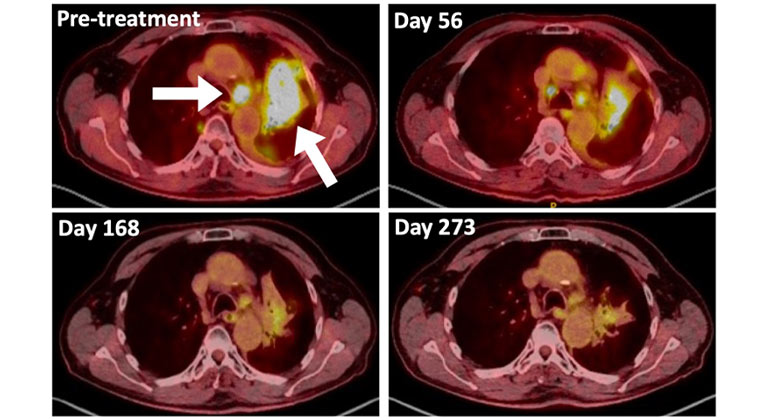
A Type of Allergy Medicine Might Help Treat Lung Cancer, Research Suggests
Dec 06, 2023 View All Press Releases