Requests to Rely (R2R) on an External IRB
Please note that all projects relying on an external IRB are still subject to all ISMMS policies and procedures for the conduct of research. Research activities may not begin until an initial acknowledgement that all requirements are met has been issued by the PPHS.
It has been our long-standing practice to work collaboratively with other academic institutions. Traditionally, we have worked with other institution’s IRB offices to determine appropriate arrangements when one institution is involved in a small aspect of the overall project. For example, if a study is recruiting elsewhere and all research interventions/interactions are occurring at that external site and our investigators are involved in the analysis of data or performing assays, we have entered into an agreements to rely on the other institution’s IRB for the review of the research.
As the research and regulatory communities have moved in the direction of utilizing a single IRB to serve as the Reviewing IRB for multi-site projects, the ISMMS PPHS Office has worked to establish a more systematic process for submission, tracking, and monitoring of these projects.
Requirements for Using the Designated Single IRB in Multi-Site Research
For multi‑site studies that utilize the R2R process, Mount Sinai IRB expects to rely on the designated Single IRB. Using a different IRB than the one selected for the rest of the study is generally not permitted, as this has the potential to introduce significant risks to participant safety, study oversight, and regulatory compliance.
Study teams are encouraged to bring their proposed IRB review arrangements to PPHS prior to beginning preliminary work with an identified external IRB to verify that the proposal meets PPHS' criteria for ceding to another IRB. Please contact the PPHS Office at 212-824-8200 or irb@mssm.edu.
NIH Single IRB Policy for Multi-site Research (posted 01.25.2018)
The PPHS has issued a statement confirming our understanding of the policy and agreeing to rely on external institutions serving as the sIRB using the SMART IRB Reliance agreement. The full statement is available for download here and may be included in grant applications meeting this criteria: ISMMS PPHS Statement on NIH sIRB Policy
R2R Guidance Documents
- HRP-232R-Request to Rely (updated 11.02.2023)
- HRP-916-Guidance-R2R Initial Submissions in RUTH (updated 11.21.2025)
- HRP-917-Guidance-R2R Continuing Review Submissions in RUTH (updated 04.14.2025)
- HRP-918-Guidance-R2R Modification Submissions in RUTH (updated 08.01.2023)
- HRP-920-Guidance-R2R NCI Initial Submissions in RUTH (posted 03.27.2023)
- HRP-923-Guidance-RNI Reporting for R2Rs (updated 10.26.2023)
- HRP-924-Guidance-CV-PI Proxy-Primary Contacts (posted 10.21.2020)
- HRP-454—CHECKLIST—RUTH R2R Continuing Reviews (posted 2.24.2025)
Please note that research activities cannot begin at any Mount Sinai location until PPHS issues an acknowledgment letter, even if the external IRB has provided their review/approval of the protocol. The PPHS acknowledgement letter documents that both external IRB approval has been obtained and all institutional requirements have been completed.
As an AAHRPP-accredited program, our policy is to only rely on Institutions who demonstrate that they have comparably robust Human Subjects Research Protection Programs. We will enter into agreements to rely on another IRB only if they are accredited.
All human subjects research being reviewed by an external IRB, through either a master reliance agreement with a commercial IRB, the SMART IRB Reliance Agreement, or through study-specific reliance agreements with other institutions, must submit a Request to Rely (R2R) to the PPHS office through RUTH for registration and review of local requirements (e.g. conflict of interest, education requirements, state law, institutional policy, Ancillary Reviews).
HRP-232R: HRP-232R-Request to Rely is a tool for study teams to assist in the development of a complete and accurate R2R submission. This document is required for all R2R submissions in RUTH.
Reliance Forms: Forms required by the external IRB (e.g. agreements, waiver of jurisdiction forms, local context forms) will not be completed prior to a submission in RUTH. While each external IRB may have specific requirements, the use of any external IRB by Mount Sinai researchers should follow the general R2R submission process:
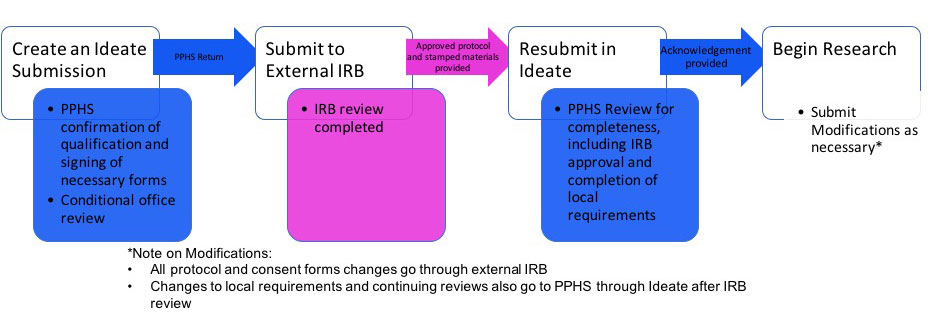
Consent Forms: All consent documents used to enroll participants in research at ISMMS are required to include site specific template language: Please click here for the site specific consent language required for ISMMS consent forms.
Once the Reviewing IRB has issued a continuing approval, that approval must also be registered in RUTH so that the PPHS can track ongoing research.
R2R continuing reviews require ISMMS GCO and IF submissions be made prior to the submission of the continuing review registration to the PPHS. This applies to all R2Rs except BRANY studies when BRANY negotiated the contract.
Financial Conflict of Interest Review
For R2R continuing reviews submitted after July 19, 2019, study teams will be responsible for initiating the FCOI Review for continuing review submissions when relying on an external IRB. It is recommended that the FCOI Review is complete prior to submission of the continuing review to the external IRB. An incomplete continuing review submitted to PPHS will be returned to the study team to complete the FCOI process. Please refer to the HRP-917-Guidance-R2R Continuing Review in RUTH for specific steps.
Continuing Research Activities
It is noted that continuing review approval from external IRBs are often received by the study team very close to the expiration date. As such, once the approval letter from the external IRB has been submitted to the PPHS, the submission will be pre-reviewed and a comment will be added to RUTH when permission has been granted for research activities may continue. Please note it is possible that modifications may be required based on local requirements/ancillary office review. A comment will be added in RUTH once local requirements have been confirmed as complete.
Once all local ISMMS requirements have been satisfied, the IRB approval dates will be set to match those issued by the external IRB.
Modifications under the purview of an external IRB do not need to be submitted to the ISMMS PPHS unless they also affect local requirements (e.g. change in PI, change in recruitment procedures, change in an ancillary office review – RSC, IDS, FACTS, etc.). Fill out the HRP-388 Modification Ancillary Review to determine if an ancillary review is needed.
The most recent versions of all study materials should be included at the time of Continuing Review registration.
Launched in 2016, SMART IRB is currently funded by the NIH Clinical and Translational Science Awards (CTSA) Program, grant number UL1TR002541-01S1. For more information, please visit www.smartirb.org.
We are a participating institution in the SMART IRB Master Common Reciprocal Institutional Review Board Authorization Agreement (SMART IRB Agreement), which provides a standard agreement and agreed upon standard operating procedures in support of the NIH single IRB mandate. The PPHS has experience conducting the necessary local context reviews and working with other institutions as a Relying institution.
Please note that the SMART IRB Agreement is an agreement, and not an actual IRB that will conduct review of the project. Each reviewing and relying institution are responsible for ensuring that the protocol-specific details of the agreement are clear for each project, as necessary. All projects utilizing the SMART IRB agreement must be submitted via RUTH as per the R2R process outlined above.
An AAHRPP-accredited Commercial IRB with which ISMMS has an existing Master Agreement, including BRANY and the NCI CIRB, may be used when 1) the use of the commercial IRB is mandated, or 2) when all of the following criteria are met:
- Multi-site, industry sponsored, study, where ISMMS is involved as a site with no additional responsibilities beyond those of other sites,
- Industry holds the IND/IDE and initiated the protocol, and
- Research is not a Phase I first-in-human study.
- Research is not a registry
- The study is not planned emergency research
When using BRANY:
- Permission to use the BRANY IRB must be obtained via a submission in RUTH prior to submission to BRANY for review.
- At the time of your RUTH submission, please ensure that the HRP-232R indicates whether BRANY is both serving as the Reviewing IRB and negotiating the contract, or whether it is only serving as the Reviewing IRB. If BRANY is not negotiating the contract, a regular GCO submission and IF are required through the usual ISMMS process.
- The HRP-232R will be signed and provided via RUTH after the initial review by the PPHS and Ancillary Offices. The signed HRP-232R serves as your permission from PPHS and must be submitted to BRANY to initiate the review.
When using WCG, Advarra or Alpha IRB:
- A consent template with the ISMMS required language already incorporated (based on the HRP-232R) must be submitted at the time of your initial RUTH submission.
- The HRP-232R will now serve as the IRB Waiver of Oversight. It will be signed and provided via RUTH after the initial review by the PPHS and Ancillary Offices. The signed HRP-232R serves as your permission from PPHS and must be submitted to external IRB to initiate the review.
- There is no longer a separate IRB waiver for WCG, Advarra and Alpha.
- Additional Resources:
When using Sterling IRB:
- Contact PPHS to obtain the Sterling IRB Jurisdiction Form.
- A consent template with the ISMMS required language already incorporated (based on the HRP-232R) must be submitted at the time of your initial RUTH submission. The bottom of the waiver will be completed once the language has been deemed appropriate.
When using Jaeb IRB:
- If you are using the Jaeb IRB - an Attachment A, specific to your protocol, and a consent template with the ISMMS required language already incorporated (based on the HRP-232R) must be submitted at the time of your initial RUTH submission.
Please contact the PPHS Office at 212-824-8200 or irb@mssm.edu with questions about the Request to Rely (R2R) requirements or whether your protocol qualifies to use the R2R process.